A recent, lengthy, exchange with a climate denier and several more after the California governor’s announcement has given me a bit of insight to Anthropogenic (man-caused) Global Warming (AGW) deniers. Many of them freely admit that there is some warming, but not that humans are the cause of said warming. I’d like to take a bit and just kill that notion right now. This involves some science, so bear with me.
Carbon dioxide (CO2), or fixed air as it was known at the time, was first hypothesized in the 17th century. But it wasn’t until the 1750s that scientists were able to isolate it, form it, and observe the properties. Those properties involve putting out candle flames and killing animals who breathed significant amounts of it.
The molecule itself is very simple, just two oxygen atoms attached by double bonds to a carbon atom. Carbon, as John Stewart says, is the slut of the Periodic Table and will hook up with almost anything. The molecule looks like this:
O=C=O
This is called a symmetric molecule, for obvious reasons. But that’s important because symmetric molecules tend to have vibrational bands in the infrared spectrum. What this means is that CO2 absorbs light at a specific frequency. It absorbs that light and begins vibrating. This causes frictional effects with other molecules around it, which in turn heats things up.
You can see this same thing happening in your microwave oven. Water absorbs (among other things) a specific frequency of microwave radiation, which causes vibrations, which causes frictional heating. While the molecules we’re talking about are very small, there are a lot of them. You can boil water in under a minute in a powerful microwave oven.
The vibrational frequencies of carbon dioxide are non-controversial. It’s an experiment that is done in college physics and chemistry labs (PDF). The earliest reference I can find is a table from 1972 in Shimanouchi, T., Tables of Molecular Vibrational Frequencies Consolidated Volume I, National Bureau of Standards, 1972, 1-160. Addendum, I also found this reference from 1969… PR Davies, WJ Orville-Thomas “Infrared Band Intensities and Bond polaritites Part1. Bond Moment constants in CO2, OCS, CS2, CSe2, and SCSe” J. Mol. Struct. 4 (1969) 163.
So, if someone rejects this, then they need to be able to prove that every science experiment and observational result is wrong or they are simply rejecting science and can be safely ignored.
What does this mean for Earth? When sunlight hits an object, that object absorbs some of the visible light energy. Some of that light is reflected back and gives our eyes information about the color of the object. The rest of the light is absorbed and causes molecules in that object to vibrate and, you guessed it, causing frictional effects which result in an increase in temperature. Some of that energy is radiated as infrared energy, heat.
Glass has some neat properties. The glass windshield of the car is transparent to visible light. Otherwise, it would be pretty useless as a windshield. It also blocks UV light, which is good and bad. You can’t get a sunburn while sitting in the car with the windows rolled up. But if you have glasses that get darker in the sun, they won’t work either, since UV light is what activates the darkening.
Glass also block infrared. So, the light emitted by objects when they are hit by visible light stays in the car. Your car is absorbing and retaining a lot more energy than can leave the car. Plus, the water vapor and carbon dioxide in the car’s interior also absorb that infrared energy and vibrate, causing more friction.
Again, this is pretty trivial to see.
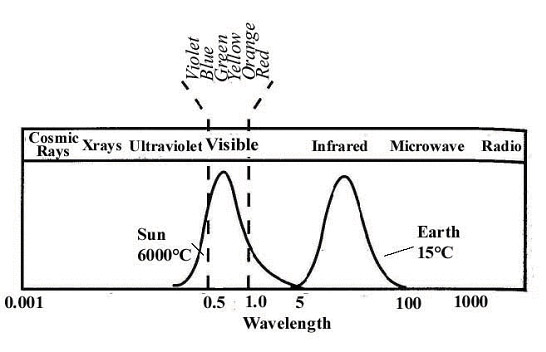
What does this all mean for our planet? Well, Earth is like a giant car. Our atmosphere is very clear to certain frequencies of light and almost opaque to others. The picture below shows how our atmosphere absorbs or transmits various frequencies of light.
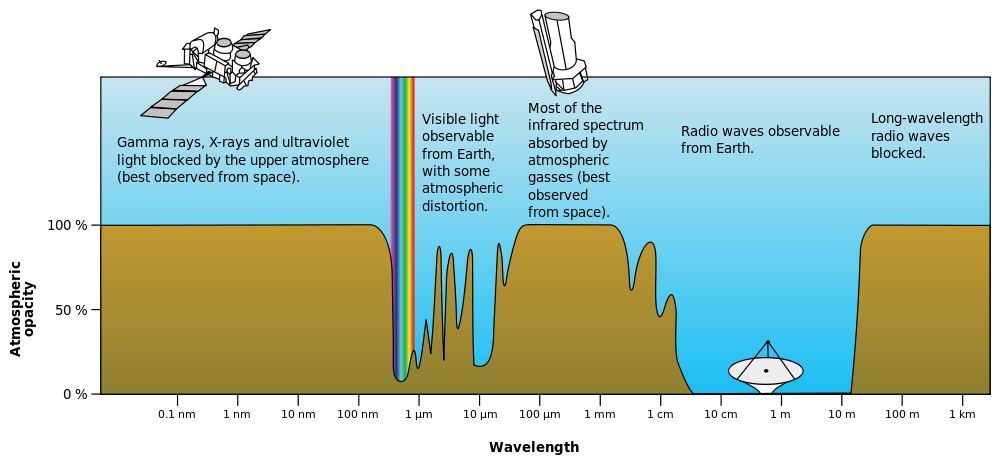
The following diagram shows where the sun emits the most light (in the visible spectrum) and where Earth emits the most (in the infrared spectrum). These two show us that most of the energy hitting Earth comes from visibly light, short infrared, and microwave (radio) radiation. All of these hit the surface of Earth and are absorbed just like light hitting your car seats. Re-emitting infrared radiation. Which, is absorbed due to the gases in the atmosphere.
Now, any climate denier will tell you that some carbon dioxide in the atmosphere is a good thing, and I and scientists agree with them. We need some carbon dioxide, otherwise Earth would be really cold… everywhere… all the time. How cold? That’s an interesting question, one answered by Lacis et. al. in 2010 (PDF). One of the things they investigated in this paper was “what would happen if all the carbon dioxide were removed from the atmosphere?”
During the first year alone, global mean surface temperature falls by 4.6°C. After 50 years, the global temperature stands at –21°C, a decrease of 34.8°C. Atmospheric water vapor is at ~10% of the control climate value (22.6 to 2.2 mm). Global cloud cover increases from its 58% control value to more than 75%, and the global sea ice fraction goes from 4.6% to 46.7%, causing the planetary albedo of Earth to also increase from ~29% to 41.8%. This has the effect of reducing the absorbed solar energy to further exacerbate the global cooling. After 50 years, a third of the ocean surface still remains ice-free, even though the global surface temperature is colder than –21°C. At tropical latitudes, incident solar radiation is sufficient to keep the ocean from freezing. Although this thermal oasis within an otherwise icebound Earth…
So, yes, some carbon dioxide is critically important to maintaining the planet’s temperature. In fact, that’s what the paper says.
Ample physical evidence shows that carbon dioxide (CO2) is the single most important climate-relevant greenhouse gas in Earth’s atmosphere. This is because CO2, like ozone, N2O, CH4, and chlorofluorocarbons, does not condense and precipitate from the atmosphere at current climate temperatures, whereas water vapor can and does.
Now, we need to say that this isn’t just chemistry, models, and theory. Let’s talk about the physical evidence of this.
The first instrumental confirmation came from an examination of the data from two different spacecraft flying 27 years apart. In 1970-71, NASA flew the Infrared Interferometric Spectrometer (IRIS) aboard the Nimbus 4. While in 1996, the Japanese ADEOS satellite carried the Monitor of Greenhouse Gases (IMG). These satellites were Earth imaging satellites. They were pointed at Earth, using instruments to record data about Earth through its atmosphere. This Nature letter describes the comparison of the data (PDF).
First the authors compared the data of an area of the central Pacific to see if the data sets were even similar. Suffice to say that, even though the two instruments were decades apart in technology, their capabilities are comparable and easily matched.
Second, the authors compared the spectrums of the two instruments for the same areas during the same months. By using a computer simulation and ground based gas concentration data, they were able to generate the expected spectra from 1970 and 1997.
We note that the main features of the observed difference spectrum can only be reproduced by including the long-term changes in CH4, CO2, O3 and the chlorofluorocarbons: inter-annual and short-term variability is not sufficient.
In other words, the only way to match the observed data was to use increased values for the primary greenhouse gases; methane, carbon dioxide, and ozone.
This means that the transparency of Earth’s atmosphere to certain wavelengths of light has decreased, specifically in the wavelengths that are blocked by carbon dioxide, ozone, and methane. Which, in turn, means that there are more of these gases in the atmosphere and they are blocking more radiation from leaving Earth.
This is direct evidence that the increase in greenhouse gases is causing warming. This isn’t a theoretical model or simulation. This is all observed data and physical and chemical fact.
I must talk about the elephant in the room. It should be clear that carbon dioxide is a greenhouse gas. Methane is as well and is significantly more potent a greenhouse gas (about 70 times stronger than carbon dioxide), but methane only last a few decades in the atmosphere, then breaks down into carbon dioxide which can stay in the atmosphere for centuries.
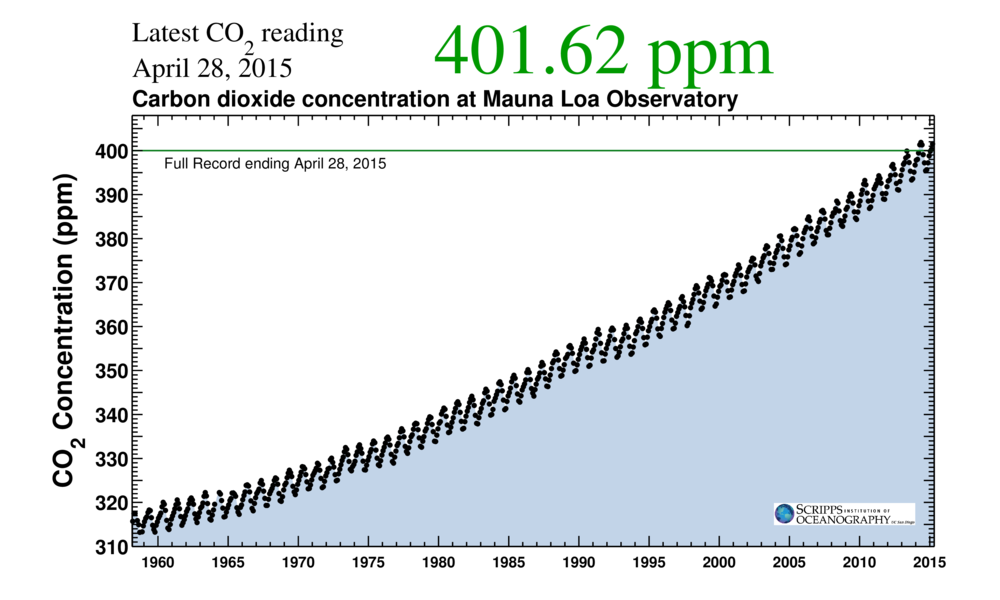
There is a twitter feed (Keeling curve) you can follow that, almost daily, will tell you the amount of carbon dioxide in the atmosphere as measured at Mauna Loa by the Scripps Institution of Oceanography. In 2013, I watched the rise above 400ppm for the first time in recorded history. From March to June of last year, the readings stayed above 400ppm (it’s always higher in the summer). So far in 2015, it’s been generally well above 400ppm, just recently hitting almost 405 ppm.
You have probably seen this curve (or one like it before).
This too is recorded instrument data, taken frequently throughout the day and averaged for hourly, daily, weekly, and monthly totals. You see all the curves and data here.
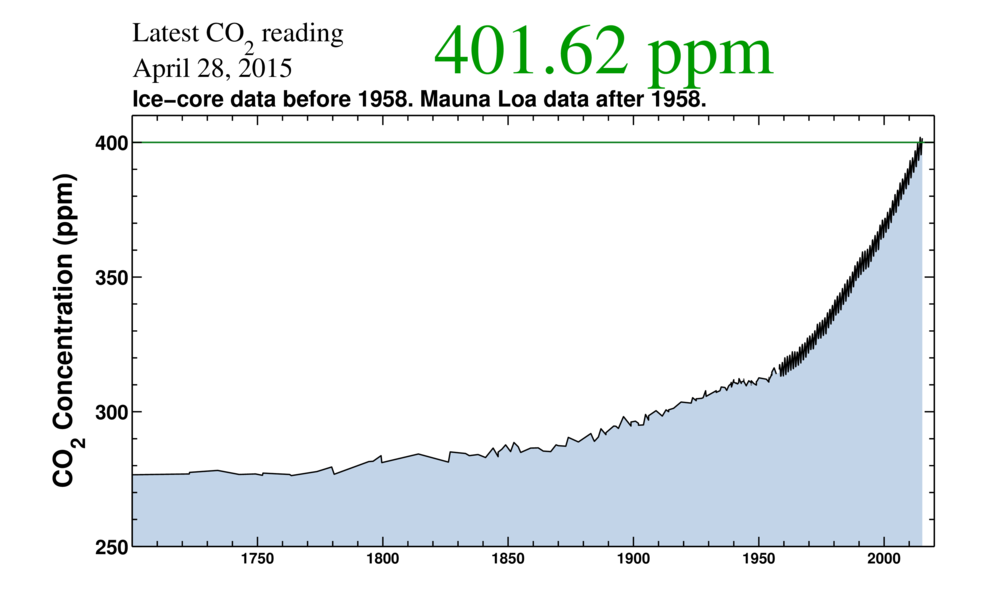
You might have also seen this curve.
As stated in the picture, the data prior to the beginning of instrument readings is estimated by using ice core data. A detailed description and reference for the ice core data and processes are here.
Why the sharp rise in carbon dioxide? Welcome to the human revolution. It’s us.
The carbon cycle is pretty delicately balanced, with carbon being used by some organisms at about the same rate as being produced by other organisms. Sure, there’s some events that change it. Volcanoes spew carbon dioxide (among other things) and peat bogs and the like absorb carbon dioxide and bury it. When I say delicately balanced, I mean it. For example, respiration (organisms breathing out carbon dioxide) results in 119.6 gigatons of carbon going into the atmosphere every year. But gross primary productivity (i.e. photosynthesis) takes about 120 gigatons of carbon out of the atmosphere each year.
Except for fossil fuels. Humans are taking carbon out of the ground and burning it.
We all know that carbon in fossil fuels comes from deep under the ground. Oil, coal, and natural gas formed tens to hundreds of millions of years ago from peat bogs, forests, and swamps. That carbon was removed from the Earth’s carbon cycle millions of years ago. It was buried deep. Fossil fuels don’t have any system in place for taking carbon out of the atmosphere, so it accumulates.
And humans, for the last 100 years, have been sucking up all that carbon and converting it into atmospheric carbon dioxide. In just 10 years, we have burned enough fossil fuels to be responsible for full 10% of the entire atmospheric reservoir (about 75 gigatons of carbon).
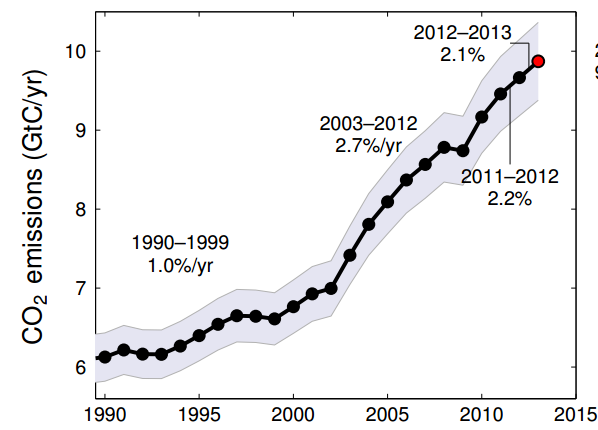
This graph shows the mass of carbon humans are injecting into the atmosphere each year. While the overall rate of growth is slowing, the amount is still increasing. It’s just not increasing as fast as it was. I should point out that there’s two ways to measure this: gigatons of carbon and gigatons of carbon dioxide. Because carbon dioxide has two extra oxygens attached, it’s heavier than just carbon. In terms of carbon dioxide, we’re talking about 30-35 gigatons per year (depending on which year). Taken from the Global Carbon Budget.
We all know that 2014 was the hottest year on record. So far, 2015 is breaking records of its own. We also know that 9 of the last 10 hottest years on record have been in the last 12 years.
Here’s a fun game to play. When was the last record cold year? Think about it a minute before reading on.
.
.
.
1911… 0ver 100 years ago.
So, there you have it.
To reject the claim that humans are causing the global temperature to increase is to reject reality.