Some people have asked for a description of fusion power and why it’s better than fission. First, let’s talk about our current nuclear reactors.
Nuclear fission is the process of radioactive isotopes splitting into smaller pieces. This isn’t a chemical reaction like steel rusting or vinegar and baking soda fizzing. In a fission (any nuclear) reaction, the elements are actually changing.
Every element has both stable and unstable (radioactive) versions. The number of protons in the nucleus is the same, but the number of neutrons vary. The tendency is for isotopes with more neutrons to be unstable.
In nuclear power plants, the one of most common isotopes used for fuel is uranium-235. The “235” is the mass. Since the number of protons for an element is constant, the mass allows you to determine how many neutrons are in the nucleus.
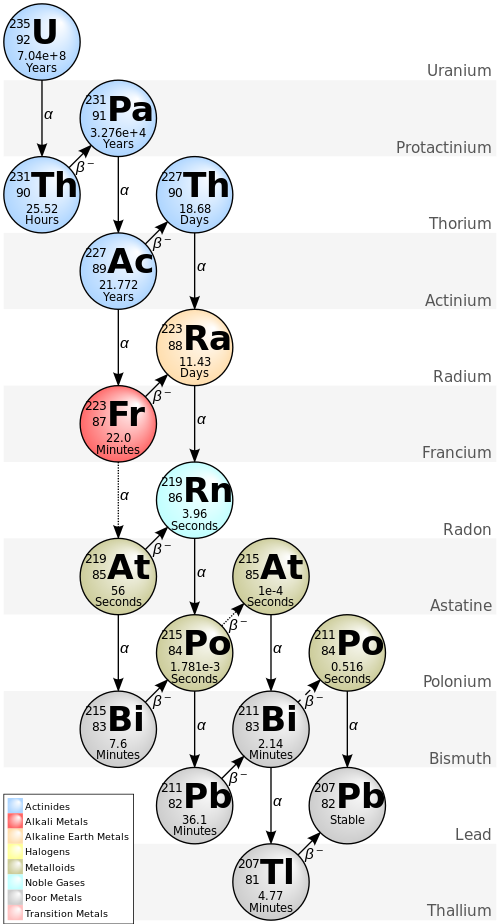
This is the decay series for uranium-235.
http://commons.wikimedia.org/wiki/File:Decay_Chain_of_Actinium.svg
There are several things in this diagram (it’s a cool diagram, right?). First of all, on the right, are the element names.
Inside the circles are the atomic mass (upper number), the number of protons bottom number) and the symbol for the element. The top one in this chart is Uranium, with a mass of 235 and 92 protons (therefore it has 143 neutrons).
Below that in the circle is the half-life. This is a strange concept, but it’s the time it takes for half of a given mass of of the element to decay (to the next one in the chain). This is a probable value, it might be slightly less or slightly more.
In the case of uranium-235, that half life is 704,000,000 years. Yeah, 700 million years.
The lines between the circles (solid and dotted) show what kind of radioactive decay happens (or can happen) to that isotope. A single solid line always happens, while a solid line and a dotted line show the possibilities. The solid lines are much, much more likely and the dotted lines are very rare events.
The Greek letter indicates what type of radiation is happening. The α is for alpha decay. In this case, the nucleus emits a particle called an alpha particle, which is really the nucleus of a helium atom. That is, two protons and two neutrons. When this happens, the atomic mass drops by 4 and the atomic number drops by two (transforming the atom into a new element).
The β– is a little more complicated. That’s beta radiation and the nucleus emits a beta particle. That is, an electron (hence, the minus sign). But the nucleus doesn’t contain any electrons. What actually happens, is a neutron breaks into a proton and an electron. I say “breaks” in the interest of ease of understanding, I don’t want to get into gluons and quarks here.
The atomic mass of the atom stays the same, since an electron is negligible compared to the protons and neutrons. But the atomic number increases since there is an additional proton now. That’s indicated by an arrow pointed upward on the chart.
As you go through this, you see that some of the radioactive products in this chain have very long half-lifes (millions of years) and some have very short half-lifes (small fractions of a second). Also note that all paths lead to the stable isotope of lead-207.
This is the decay series. Since humans are getting involved, we just throw most of that away.
What humans do is put some uranium-235 in a reactor. Different types of reactors need different percentages of U-235. Some require less than 5% of the fuel to be U-235 (with the rest being relatively stable U-238). What happens in fission reactors is a stray neutron, generally from another fission event, hits a U-235 nucleus. This (after some extremely rapid changes to the nucleus) causes the U-235 atom to split into an atom of barium-141, krypton-92, and 3 more neutrons. Those neutrons go on to strike other U-235 atoms, causing similar reactions.
When the atom fissions, a large amount of energy is released. It’s about 80 Terajoules per kilogram of fuel fissioned. Those are kind of abstract figures to most people. A terajoule is about 30 years of electrical energy use for the average home.
Most of that energy is lost to heat and only a fraction is actually converted to usable electricity. The actual electrical energy generated is about a terajoule per 20 kilograms of material. But still, that’s a lot of energy for a single kilogram of material.
If this process is not controlled, then each neutron will generate 3 more, resulting in a chain reaction. More and more of the U-235 fissions. This generates lots of heat and could eventually cause a meltdown event. This is where the reaction can no longer be contained or controlled. The heat increases beyond what is containable (and can be enough to burn through concrete).
Normally (and bad fission events are actually quite rare), the number of neutrons is controlled. There are several materials that absorb the neutrons. Nuclear power plants can adjust the control to increase or decrease the number of fission events, thereby creating an ideal amount of energy. The heat energy is then transferred to steam turbines just like in a coal or oil-fired power plant.
The down side of all this, is that in a few years, the fuel will be used up to the point where it cannot sustain reaction. There’s too much non-fissile material. Unlike oil or gas or other power plants. When the nuclear fuel is not capable of continuing the nuclear reaction, there is still a fair bit of U-235 left. Which, as we saw above, will still be radioactive for another seven million years or so. And will decay in the process above and neutron fission into the various isotopes listed.
Of course, barium-141 and krypton-92 are also radioactive with decay processes of their own and one of krypton-92 decay processes releases a neutron.
That all sounds quite scary. But because nuclear fission reactions are so powerful, the amount of fuel needed to actually run a reactor for years is quite small.
Each year, the total amount of nuclear “waste” generated by every nuclear reactor on the planet is only about 2,000 metric tons. Over the past 40 years, the total amount of nuclear waste is only 71,780 metric tons. Which sounds like a lot, but nuclear fission fuel is so dense, that this would amount of waste would basically cover a football field to a depth of 21 feet.
In comparison, the fossil fuel power plants (world-wide) release of 10,000,000,000 tons of CO2 per year. The top twelve worst CO2 polluters in the US each generate more than 16 million tons of CO2 per year. But we don’t notice that because it goes into the atmosphere. Note that this isn’t a totally fair comparison, because we have to consider percentage of waste per kilowatt hour or some other valid comparison. Still, over 10% of all electrical energy in the world is produced from nuclear power plants. And increasing that to 100% would mean only about 20,000 tons of nuclear waste per year instead of tens of millions of tons of CO2.
Still, that’s a lot of radioactive by products. What if we could get energy out nuclear reactions without all the radioactive waste?
That’s the goal of fusion power.
Fusion is a much simpler nuclear reaction. Instead of breaking a large atom into smaller pieces, the goal is to take some of the simplest atoms (and most common) in the universe and fuse them together into larger atoms.
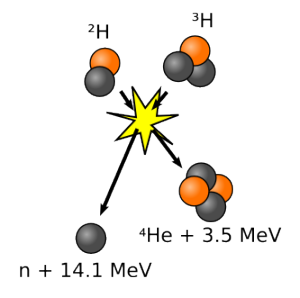
In this, we take two isotopes of hydrogen (deuterium which is a proton and a neutron and tritium which is a proton and two neutrons) and fuse them into a helium nucleus and energy. It also releases a neutron and energy.
This is actually less than the energy released by U-235 fission, but the downsides are greatly reduced. There is a problem with that high energy neutron. It can hit the reactor vessel walls and cause the atoms in that material to become radioactive. But that’s a very minor issue. It might mean that the reactor vessel has to be replaced every few years.
In terms of waste, this is very clean. Helium actually has some useful properties and if it can be extracted, then could potentially be sold as well. Deuterium is a naturally occurring isotope of hydrogen and must be extracted from water, but this a common and well understood process. It’s also likely that it is quite common on other planets and the moon. Tritium is even more rare on Earth and also more abundant on the moon (PDF). Interestingly, tritium can be a byproduct of fission reactors and is formed naturally in the atmosphere.
Still, there’s a lot of this stuff around.
The problem is that fusion can only occur (so far) at high temperatures. We’re talking core of the sun type of temperatures. And high pressure. Core of the sun type of pressure.
These condition are difficult to create on Earth. It can be done very briefly, but we need a constant and continuous fusion for power generation.
That’s what all the research is for and trying to achieve. There are a variety of possible methods. Most of them involve using superconducting magnets to create a bubble of magnetic force. Since the atoms at those high temperatures don’t have electrons, they are positively charged. A magnetic field can contain the ions. One method of increasing the temperature and pressure is to use that magnetic bubble to compress the ions. That takes a lot of energy though. So far, it takes more energy than the reactor can generate.
Another method is to create a torus and accelerate the ions to high speed within that torus. Speed can replace temperature and pressure causing the ions to hit fast enough to fuse. Again, this is massively energy intensive. It’s what ITER is planning to use.
My understanding is that Lockheed-Martin’s project will be using the magnetic compression bubble method or something similar.
In either case, the heat generated by the fusion will boil water, generating steam, which is used to drive turbines. Just like a fission plant or a coal or oil power plant.
At this point, no one is really sure how much energy can be extracted from a fusion plant. There are some different types of possible fusion reactions as well. They are similar in principle, but use different combinations of hydrogen isotopes for fuel.
That’s the basics of fission and fusion power.
