I cannot believe that we are still trying to convince creationists that the Second Law of Thermodynamics (SLOT) doesn’t somehow prevent evolution from happening. (BTW: Please don’t visit the link. It’s to an internet cesspool of creationists all patting each other on the back about how no one can argue with them. Of course, the massive bans every time someone dares to object couldn’t have anything to do with it. Please, let Uncommon Descent die the long, slow, painful (embarrassing) death it deserves.)
Anyway, because this keeps coming up (even though Answers In Genesis says not to use it), I’ll put a refutation here so anyone can refer back if needed. I will be gentle, so even if you don’t like physics, you can handle it.
First, what does the Second Law of Thermodynamics say:
A change in the entropy (dS) of a system is the infinitesimal transfer of heat (δQ) to a closed system driving a reversible process, divided by the equilibrium temperature (T) of the system.
It can be written like this:
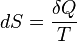
Now, that all looks scary (even to me), so let’s talk about it in pieces.
A change in entropy
What is entropy? First of all, what is it not? Entropy is NOT order or disorder or anything like that. If you ever see anyone say that ‘entropy’ is disorder, then you are free to ignore them as they do not have a clue what they are talking about. Most creationists fit into this category.
Entropy is a measure of the amount of thermal energy per unit temperature that is unavailable to do work (in the physics sense). Entropy is a measurable, calculable concept. It is not some vague hand-wavy thing that people talk about in hushed tones. Since entropy is measured in energy/temperature, then it has a unit, Joules per Kelvin (J/K). So, there’s another good test of the person you’re talking to. If they can’t give you the units of entropy, then they don’t have a clue what’s going on.
A brief aside: Analogies are a good way to teach people who don’t have the needed background for a concept. Analogies are not the concept themselves.
So, when the entropy changes, it means that the amount of work available changes, either increases or decreases. And yes, entropy can increase. We’ll get there in a minute.
of a system
Physicists talk about ‘systems’. A system is a group of pieces or components that interact. They can interact mechanically ( a car is a system). They can interact chemically (a reaction in a test tube is a system). They can interact using energy (the Earth and Sun is a system).
Now, when physicists talk about systems, there are several things to keep in mind. A system has a boundary. Everything inside that boundary is the system and everything outside is the environment. They do interact, but we have to draw the line somewhere.
There is a physics concept called a “closed system”. This means that the system does not interact with the environment at all. This is an unreal concept. There really isn’t such a thing as a ‘closed system’, because all systems interact with their environment, even if they have been specially designed to reduce that interaction as much as possible (like a thermos bottle). An ‘open system’ is one in which the system can interact with the environment.
is the infinitesimal transfer of heat (δQ)
Q is heat in physics. Heat is energy. It is measured by a thermometer which measures the kinetic energy of molecules in a substance. As the heat (energy) of that substance increases, the molecules/atoms move more quickly and the temperature rises.
It is possible, through interactions, for heat in one area to move to another area. You can heat up one column of air, then push that air through a house to warm the whole house. You can turn on a radiant heater which produces infrared radiation, which transfers heat energy.
So, basically, the second law of thermodynamics (so far) is talking about how heat energy moves in a system.
to a closed system
Here’s that system thing again. A closed system, remember, doesn’t actually exist. It’s just easier to talk about it this way,
driving a reversible process
A reversible process is a very specific physics term. It doesn’t mean “if you break it, you can put it back together”. It means that a process can be reversed without a change in energy of a system. That last bit is very important. For example, if you compress a fluid (say air), then the temperature increases. If you then decompress the fluid, the temperature decreases again. Allowing for losses due to friction, you will end with the same energy you started with. That is a reversible process.
Mixing something like sugar and sand is not a reversible process… in physics. Because it requires a lot of energy to separate the two again.
divided by the equilibrium temperature (T) of the system.
Equilibrium is another very specific concept in physics. It is the state where the energy and material flow into a system is exactly the same as the energy and material flow out of a system. The equilibrium temperature is (shockingly) the temperature at which the system is in equilibrium.
Again, in reality, there are very few (if any) systems that are in true equilibrium.
OK, so that’s the definition of the 2nd Law of Thermodynamics (and entropy for that matter). Notice that there is no discussion of order/disorder in there.
In simpler terms, SLOT says, in a closed system, the energy available to do work will always decrease.
Rudolf Clausius said “The entropy of the universe tends toward a maximum.” This is one of the more well known statements of SLOT. Unfortunately, it is not really true. Without defining the terminology (like ‘universe’) and whether the system is open or closed, the phrase “tends toward a maximum” has no meaning.
Now creationists will often use that to say that “information can’t increase’ or support their Flood mythology using SLOT (e.g. everything is winding down, so man has been falling ever since the garden of Eden) or whatever. That’s not what SLOT is saying. Let me give you an example that completely refutes everything the creationists have said about or using SLOT.
Every cell in your body requires energy. Known fact. If the cell doesn’t get enough energy in the form of ATP, it cannot do all the things it needs to (pump in ions, pump out waste, assemble proteins, copy DNA, etc.). Each cell in your body is a system. Since the entropy of a system increases toward a maximum, then the cell will eventually run out of useful work (entropy) and die. Pretty quickly really.
But cells aren’t closed systems. They take in nutrients and energy (in the form of glucose) from outside the cell. Since chemical energy is coming into the cells, they are not closed systems and the entropy can increase. In fact, the entropy can decrease so much that the cell can copy itself.
The same with your entire body. If your body was a closed system, then your birth would be the point in your life where you had the most energy available to do work. But we all know that’s not correct. You grow up. My son is 40 pounds and almost 40 inches tall. I’m almost 200 pounds and 72 inches tall. But I’m 35 years older than my son, how is that possible?
Because we are not closed systems either. We take in energy in the form of sugar from the plants and animals that we eat. Since we are not closed systems, then SLOT doesn’t really apply and we are free to increase our energy (decreasing entropy) as much as we like.
Where do the plants get their energy? The sun. The Earth is not a closed system either. We’ve got that big ole fusion furnace that’s fusing 620,000,000,000 kilograms of hydrogen per second, generating 3.846×1026 Watts (the equivalent of 9.192×1010 megatons of TNT) every second. That’s a lot of energy… and a small portion of that energy is impacting the Earth every second. And that miniscule percentage of energy is enough to drive all the processes and systems that we know and love (like the cat trying to get me to pet him right now).
Yes, the sun’s entropy is increasing. It’s ability to do work is slowly decreasing. In time, the hydrogen density in the core will decrease to the point where fusion isn’t possible, then all kinds of nasty things will happen, but we will have been for billions of years at that point. However, while it is pumping out that energy, other systems are able to harness that heat and use it to do work.
At some point in deep future time (tens of trillions of tens trillions of years), the universe will have run out of the ability to do useful work. There will be no stars, no black holes, nothing that even looks like a proton or election, much less an atom. At that point, the entropy of the universe will be a maximum. Everywhere in the universe will be exactly the same temperature. That is what we call the heat death of the universe.
Until then, heat can be converted into work. It can be moved from system to system. And as long as there exists a place with a higher temperature and a place with a lower temperature, heat will flow (insert Arrakis joke here), and work will be done in the universe.
