Update: Here is the link to the original Guardian piece from which most of this post was quoted.
In the second part of my posts on how evolutionary biology impact medical science, and hence how creationism undercuts human health and endangers lives, I will be talking about Hepatitis B. Where did this dangerous virus come from? And how does it cause liver cancer? Scientists are beginning to shed light on these questions.
Hepatitis B (belonging to the hepanda virus family) is an odd ball. It uses DNA, and not RNA like some other viruses, as the molecule to carry its genetic code. Nonetheless, it goes through the cycle of copying its DNA into RNA inside the host cells, and subsequently copying it back into DNA, which it then integrates into the host cell genetic material. To do so, it uses an enzyme called reverse transcriptase. This step is very much like what retroviruses (such as HIV) do: they copy their genetic material, in the form of RNA, into DNA and then insert it into the host genome. Which is how we know hepatitis B and HIV are evolutionarily related. It is also the reason certain medications (such as lamivudine) can be used to treat both conditions.
Now, evolutionary geneticists have known for some time that retrovirus DNA sequences can ultimately lose their infectious abilities, and essentially become harmless, while still being passed on from generation to generation. Tracing back these retrovirus relics can help find common ancestry among different species. As it happens, the same it true for hepatitis B. Sequencing hepatitis B genes in bird genetic material gives us a clue about the ancient virus that infected the common ancestor of several different bird taxa.
Although viral genomes evolve rapidly, their rate of change slows to the same pace as that of the host’s DNA after insertion, making it possible to study viral DNA sequences that are many millions of years old.
“The prehistoric viral DNA becomes frozen in its original state at the time of insertion into the host genome and thus remains discernible as such until present”, said Dr Jürgen Schmitz, an evolutionary biologist at University of Münster and co-author on the paper.
By identifying, sequencing and analysing these genetic fingerprints, it is possible to reconstruct viral ancestors and learn more about them. This new field of study is known as paleovirology.
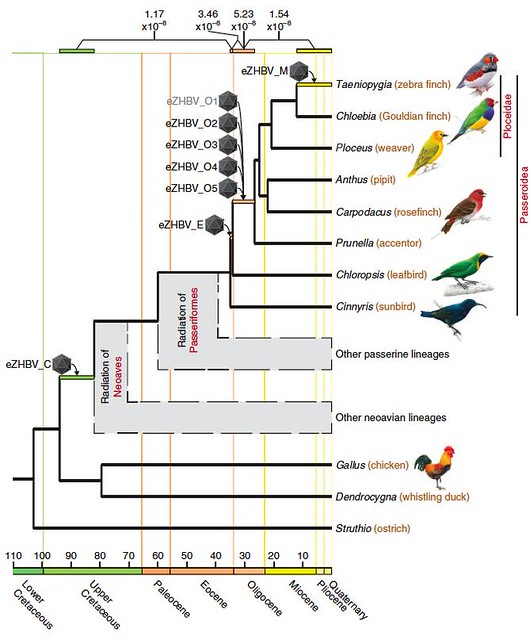
In their work, Dr Suh and his colleagues sequenced and compared the viral fragments found in the genomes of a number of songbird species. They sifted through the avian DNA in search of HBV sequences, working back in evolutionary time from close relatives to zebra finches to more distantly related birds.
They found 16 viral fragments from HBVs that had been captured 12 different times in avian genomes, and eight of these were suitable for further analysis. Since the team could calculate how long ago these birds’ common ancestors lived, they could use this information to pinpoint the age of each of these ancestral virus fragments residing in their genomes.
And the results:
According to their findings, the first ancestral HBV became trapped in avian DNA quite early: the common ancestor of all the bird species carrying this particular viral fossil lived about 82 million years ago. This indicates that the ancestral HBV dates from the Late Mesozoic, shortly after the time when modern birds (neoaves) arose and before the songbird and parrot lineages split — back when the dinosaurs were still very much alive.
Amazing, isn’t it?
But it gets better.
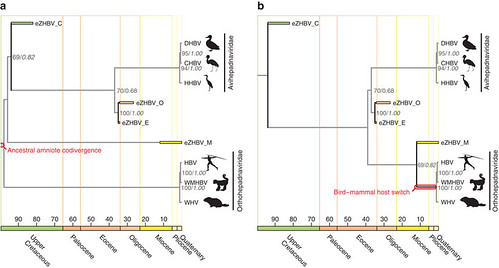
When the team rooted the hepadnaviral family tree to mammalian HBVs — a scenario suggesting that the common ancestor of this viral family may have first infected mammals — their analysis showed that HBVs infected the first amniotes and split into separate lineages more than 324 million years ago when the early bird and mammal lineages split.
Alternatively, when the hepadnaviral family tree was rooted to avian HBVs (figure 4b) — a scenario suggesting that the common ancestor of hepadnaviruses may instead have first infected birds — their analysis indicated that HBVs began infecting mammals much later. This second scenario is more likely because of the apparent absence of any HBV fragments trapped in mammalian genomes and also because of the topology of the family tree.
“HBVs have probably been infecting mammals for a much shorter time than they have been infecting birds throughout much of their evolution,” said Dr Suh in email.
“The most exciting and unexpected finding is that our oldest paleovirus is both extremely old and comprises a complete HBV genome sequence”, said Dr Suh in email.
Nevertheless, despite its age, the reconstructed Mesozoic-era avian HBV is remarkably similar to the HBV that infects people today, the team found.
“We’ve had 82 million years of evolution, but they have the same proteins,” said Dr Suh.
Update: Here is the link to the original Guardian piece from which most of this post was quoted.
So we have discovered the genetic code of a virus that infected the last common ancestor between parrots and songbirds, and can tell how similar it is to the modern day virus. Also we know that hepatitis B infected birds in their very first evolutionary days, and has been with them since-and then it jumped to mammals, including us. Such is the power of science.
But what is the utility of such discoveries?
But the team’s analysis showed one difference between the Mesozoic-era avian HBVs and the modern mammalian HBVs: the mammalian version manufactures a protein not found in avian HBVs. This is known as the X protein.
Originally, many scientists thought that avian HBVs lost the X protein during evolution, but the reconstructed ancestral HBVs didn’t show any trace of the X protein, indicating that it was never present to begin with. The X protein is necessary for HBV replication in mammals, indicating that gaining this protein was the reason that this HBV lineage switched hosts.
“The complete role of the X protein of mammalian HBVs is not known, but it has been shown to promote tumors”, said Dr Suh in email.
These findings will enhance our understanding of HBV infectivity and improve health outcomes in humans.
Understanding the origin and function of a protein that not only helps the virus replicate in humans, but how helps it cause tumors. Such is the benefit of studying evolution. And what is the use of creationism?
