With the US Supreme Court hearing arguments today about whether or not to dash California’s anti-gay marriage law (Prop 8), this seems like a good time to talk about the state of the scientific understanding of homosexuality, and in particular, the biological bases of it.
As noted by my Skeptic Ink colleague Notung recently, scientific knowledge and rationality are separate from moral judgement, which he bounded as a purely philosophical activity. Nonetheless I assert (and I believe that Notung agrees) nobody’s moral consideration process works very well without good knowledge, no matter what that process is. In my own judgement, the causes of homosexuality in humans are irrelevant to any consideration of rights or law. If it could be chosen as freely as choosing what shirt to wear today, I would still consider it anyone’s legitimate choice in the eyes of the law or under the Constitution.
Sexual preference is not a choice, though, and there are negative consequences in the form of the actions of people who believe that it is. These include “conversion therapy” Christians who believe homosexuality is a curable disease, and post-modernists views that sexual orientations can be socially manufactured which lead to the “political lesbianism” among second-wave feminists and to transphobic feminists outting of transpeople. For these reasons, and because it’s fucking interesting, I’d like to discuss the findings in biology.
 A recent Quarterly Review of Biology paper by William R. Rice, Urban Frieberg, and Sergey Gavrilets proposed an epigenetic model attempting to explain homosexuality among humans, and reviewing some of the existing literature on the topic. In this writing I will summarize and discuss the paper, Homosexuality as a consequence of epigenetically canalized sexual development. You can read the full PDF online here.
A recent Quarterly Review of Biology paper by William R. Rice, Urban Frieberg, and Sergey Gavrilets proposed an epigenetic model attempting to explain homosexuality among humans, and reviewing some of the existing literature on the topic. In this writing I will summarize and discuss the paper, Homosexuality as a consequence of epigenetically canalized sexual development. You can read the full PDF online here.
A queer evolutionary mystery
Any trait that consistently reduces the (statistically likely) number of offspring should be quickly filtered from any gene pool. And yet, Rice et al. report that about 8% of women and men are homosexual (inclusive of bisexuals). Humans are far from unique in this as homosexual behavior is documented in over 500 species. Past genetic models have been based on proposed adaptive benefits that might compensate for a homosexual individual’s reduced reproductive output. The kin selection model supposed that the support a gay person might give to their close relatives (particularly nieces and nephews) could provide a greater adaptive benefit. Alternatively, a model of “sexually antagonistic” genes asserted that genes which might make a sex especially good at mate competition also occasionally (inadvertently) resulted in homosexuality. Both approaches have failed upon analysis and empirical testing. Even the search for proximate mechanisms is a list of mostly failures.
Is there a gay gene?
Nope. In spite of many attempts to find a genetic correlate of homosexuality, there is no definitive link so far. This is even more vexing in light of the evidence that the trait is heritable and runs in families (by which I mean genetic families. Siblings raised apart are more likely to have the same orientation).
Maternal antibodies?
Researchers noted in the 90’s that gay men were more likely to have older brothers than straight men. A few years ago a theory about maternal antibodies was advanced to account for the finding. Namely, that maternal antibodies attack male-specific antigens which can pass through the placenta and affect sexual differentiation in the fetal brain. This hypothesis enjoys some empirical support, but only explains a fraction (at most 1/7) of the of gay men (those who have one or more older brother) and says nothing at all about women.
Classical hormone model is also inadequate
Prenatally gonadectomized male rodents grow into behavioral and morphological females. A similar outcome is seen in humans. Genetically XY individuals with complete androgen insensitivity (the inability of cells to respond to testosterone and other androgens) grow into physically and behaviorally female adults, despite the presence of the Y chromosome. The role of hormones is clearly important to producing male and female phenotypes, but it turns out there is much more to it than mere levels of available testosterones or estrogens.
Rice et al. point out that during gestation there is overlap in the levels of testosterone (T) among male and female fetuses in all weeks except for 15-19. This means during all other weeks, some female fetuses have as much or more T than male. Specific consideration was given to the development of the genitals. Genitals should be the most affected by androgen levels, and are among the most sexually dimorphic traits, and yet in humans and rats male and female embryos have significant overlap of T levels. At the same time, discordance between genetic sex and that sexes typical genital formation is rare. Even at the most basic physical level, hormone levels by themselves are not enough to explain developmental outcomes. The new model proposed by Rice et al. focuses on epigenetically canalized sexual development which could fit the data better.
Epigenetic canalization: Lamarck vindicated?
Canalization is a kind of robustness in which phenotypic outcomes are safeguarded against environmental and genetic vicissitudes, which are unpredictable. C.H. Waddington coined the term and conducted an experiment in which fly pupae exposed to heat shock developed a “crossveinless” phenotype in some flies. By selectively breeding them, he eventually produced flies of that phenotype even without the heat shock. The change in phenotype did not involve new mutations or any particular changes in the genome, so how did the apparent phenotype change? Through epigenetic mechanisms, but more on that in a bit.
Sexual development is canalized because many things can impact androgen availability: random genetic mutations, environmental agents, misregulation of T, and even ingested foods. One Indian medicinal herb Tinospora cordifolia is an “androgen mimic”. These could all cause tremendous problems for a developing fetus, were it not protected by canalization. The authors describe six such mechanisms, but to be brief I will describe just one. A particular enzyme can convert T into a more powerful form of T called DHT (dihydrotestosterone). However, said enzyme may not convert the T mimic (such as T. cordifolia), preventing disruption it might cause. This is just as effective for canalizing a female embryo because in the brain T is converted to estradiol (E, a common estrogen) but since the mimic will not be converted, there will be no adverse effects from it. Aromatase, the enzyme that converts T to E, may itself be a form of canalization. Now let us return to the question: if there is no change in the genes, how are these enzymes up-regulated depending on the sex of the fetus?
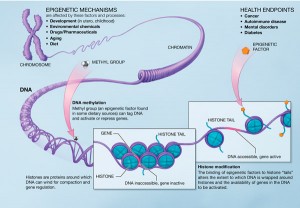
Epigenetics is the study of “changes in gene expression …caused by mechanisms other than changes in the underlying DNA sequence.” There are many ways that this can happen, but to briefly describe a few, it turns out that DNA can be bookmarked by molecules called methyl groups. The bookmarks can lead to genes being “read” more or less often. Also the protein balls called histones, which DNA spends most of its time coiled around, can have their shape altered to effect the expression of genes (see image above). Canalization (in this case) then can be understood as applying bookmarks early in embrionic development to stem cells, and selectively so depending on whether the embryo is XX or XY. Note the stem cells part. Changes made to the stem cell epigenome will be inherited by all future cells, including eventually, eggs or sperm.
According to the authors, shortly after conception an attempt is made to wipe all epigenetic modifications from the DNA. This makes a good deal of sense, as cumulative epigenetic markers (the authors use the expression “epi-marks”) could really make a mess of things over time. However, this erasure process does not erase all of the methyl bookmarks and histone modifications. What happens next is astounding: a nearly genome-wide epigenetic imprinting. Some genes are silenced as they will not be used until much later while genes necessary to stem cell functioning are up-regulated. The authors speculate that at this point, if fetus is XY, epigenetic markers could cause androgen receptors to be more sensitive and T signaling to be up-regulated in order to protect it from low androgen conditions (and the opposite for and XX fetus, to protect it in the case of overly-elevated circulating T).
Neat. Now explain the gayness and heritability
So, epigenetic canalization is well and good. Let’s imagine an XY fetus genome gets canalized as described. This leads to many changes, such as more DHT at particular times and places and greater sensitivity to T in parts of the body and the brain during both development, and the lifespan. Said individual may eventually father a daughter. His sperm cell, like all of his cells, inherited the original canalizations for maleness. The epigenetic erasure process after conception fails to erase all of the epigenetic modifications designed to canalize male not female sexual development. The fetus is still XX and will never have testes, so the individual will be female but there may have been masculinization of parts of the brain during development, resulting in homosexuality. The opposite scenario could as easily occur for an XY individual.
Rice et al. constructed a mathematical model designed to suss out the relative fitness benefit of hypothetical genes which sexually canalize a fetus during development (see the math in Appendix B of the paper). They concluded,
These calculations demonstrate that mutations causing sexually antagonistic epi-marks can invade even when the cost to the harmed sex far exceeds the benefit to the favored sex. This conclusion holds irrespective of linkage to the sex chromosomes or autosomes.
In some scenarios, the detrimental cost (lost fitness of homosexual offspring) would have to be 4 times as large as the benefit value before it would cease to be adaptive overall. Why so much? If I am understanding the model correctly, this is because of the diminishing likelihood of detrimental expression in the hypothetical grandchildren. The immediate offspring are guaranteed to have the gonad-concordant canalization. Of potential grandchildren, only half of them will be opposite-sex versus its parent (and therefore, perhaps be homosexual). The other half will gain the same benefit as the parent. This is assuming 100% heritability, which is unlikely, of course. With lower, more realistic heritability, the model favors epigenetic canalization even more strongly.
Other evidences
Astonishingly, observable physiological sex differences appear in the earliest stages of development, as soon as 5 days after conception:
[…] At this time, XY embryos are physiologically distinct from XX embryos, having a higher metabolic rate, faster growth rate, and increased resistance to some stress agents (reviewed in Gardner et al. 2010). Correspondingly, by the preimplantation blastula stage, the two sexes are reported to have widespread differences in gene expression levels at many hundreds of genes, most of which are autosomal (see Bermejo-Alvarez et al. 2010 and reference therein). Regulation of gene expression in complex eukaryotes is usually accomplished via epigenetic modifications (methylation of CpGs on the DNA or modification of histone tails;
The author’s model is consistent with the current unexplained heritability (20-50%) of homosexuality among genetically identical twins. It also explains why genetically identical twins can differ in their sexual orientation (epigenetic processes have developmental variance without regard to genes).
Other androgen-sensitive phenotypes, including cryptochidism and hypospadias “show the same pattern of high prevalence, strong familial associations, low monozygotic twin concordance, and discordance between the gonad and the trait”. There are also fitness costs associated with these conditions, and yet they also persist in the population at small but significant frequencies.
Epigenetic gene promoters have been shown to transmit across generations.
There are some studies which indicate mothers and/or female relatives of gay men are more fecund.
Conclusion
While this is a fascinating paper and an intriguing model, the hypothesis is only that. Specific epigenetic mechanisms implicated are not presently identified. It’s also not clear why epigenetic canalization is so important (if it is) that about of 8% of the population can incur a fitness penalty as a result. There are other imaginable adaptive means including regular genetic mutations or modifications to the developmental processes which could theoretically perform the same functions. I also would have liked to see discussion of epigenetic imprinting during sperm formation, and not merely post-conception. Every sperm cell bearing a Y chromosome is destined to create a male, and X a female. Therefore, they should be targets for canalized imprinting.
Whatever the causes of sexual behaviors turn out to be, all people should be treated with dignity and respect.
Homosexuality as a Consequence of Epigenetically Canalized Sexual Development
William R. Rice, Urban Friberg and Sergey Gavrilets The Quarterly Review of Biology , Vol. 87, No. 4 (December 2012), pp. 343-368

